Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 by Knudsen effusion mass spectrometry
by Knudsen effusion mass spectrometryNakajima, Kunihisa; Takai, Toshihide; Furukawa, Tomohiro; Osaka, Masahiko
Journal of Nuclear Materials, 491, p.183 - 189, 2017/08
Times Cited Count:8 Percentile:61.27(Materials Science, Multidisciplinary)One of the main chemical forms of cesium in the gas phase during severe accidents of light water reactor is expected to be cesium metaborate, CsBO , by thermodynamic equilibrium calculation considering reaction with boron. But accuracy of the thermodynamic data of gaseous metaborate, CsBO
, by thermodynamic equilibrium calculation considering reaction with boron. But accuracy of the thermodynamic data of gaseous metaborate, CsBO (g), has been judged as poor quality. Thus, Knudsen effusion mass spectrometric measurement of CsBO
(g), has been judged as poor quality. Thus, Knudsen effusion mass spectrometric measurement of CsBO was carried out to obtain reliable thermodynamic data. The evaluated values of standard enthalpy of formation of CsBO
was carried out to obtain reliable thermodynamic data. The evaluated values of standard enthalpy of formation of CsBO (g),
(g), 
 H
H
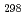 (CsBO
(CsBO ,g), by the 2nd and 3rd law treatments are -700.7
,g), by the 2nd and 3rd law treatments are -700.7 10.7 kJ/mol and -697.0
10.7 kJ/mol and -697.0 10.6 kJ/mol, respectively, and agree with each other within the errors, which suggests our data are reliable. Further, it was found that the existing data of the Gibbs energy function and the standard enthalpy of formation agreed well with the values evaluated in this study, which indicates the existing thermodynamic data are also reliable.
10.6 kJ/mol, respectively, and agree with each other within the errors, which suggests our data are reliable. Further, it was found that the existing data of the Gibbs energy function and the standard enthalpy of formation agreed well with the values evaluated in this study, which indicates the existing thermodynamic data are also reliable.
 (g)
(g)Nakajima, Kunihisa; Arai, Yasuo
Journal of Nuclear Materials, 294(3), p.250 - 255, 2001/04
Times Cited Count:4 Percentile:33.39(Materials Science, Multidisciplinary)no abstracts in English

Nakajima, Kunihisa; Arai, Yasuo; Suzuki, Yasufumi; *
Journal of the Mass Spectometry Society of Japan, 47(1), p.46 - 48, 1999/00
no abstracts in English
Nakajima, Kunihisa; Arai, Yasuo; Suzuki, Yasufumi
Journal of Alloys and Compounds, 271-273, p.666 - 669, 1998/00
Times Cited Count:10 Percentile:57.42(Chemistry, Physical)no abstracts in English

Nakajima, Kunihisa; Arai, Yasuo; ; *
Journal of Nuclear Materials, 248, p.233 - 237, 1997/00
Times Cited Count:4 Percentile:37.13(Materials Science, Multidisciplinary)no abstracts in English
 and BaUO
and BaUO in atmospheres simulating accident conditions
in atmospheres simulating accident conditionsHuang, J.*; *; Yamaguchi, Kenji*; *; *;
Journal of Nuclear Materials, 248, p.257 - 261, 1997/00
Times Cited Count:6 Percentile:47.96(Materials Science, Multidisciplinary)no abstracts in English
Arai, Yasuo; Nakajima, Kunihisa;
Proc. of 4th Int. Information Exchange Meeting on Actinide and Fission Product Partitioning and Transm, 0, p.347 - 357, 1997/00
no abstracts in English
Kudo, Hiroshi
Kagaku To Kogyo, 45(5), p.941 - 944, 1992/00
no abstracts in English
Kudo, Hiroshi
Kagaku, 46(5), 353 Pages, 1991/00
no abstracts in English
 by Knudsen effusion mass spectrometry
by Knudsen effusion mass spectrometryNakajima, Kunihisa; Takai, Toshihide; Furukawa, Tomohiro; Osaka, Masahiko
no journal, ,
Gaseous cesium metaborate, CsBO (g), is predicted to be the main cesium vapor species, depending on temperature, in severe light water reactor accidents according to thermodynamic analysis, which treats chemical reactions between boron and cesium. But quality of the thermodynamic data of CsBO
(g), is predicted to be the main cesium vapor species, depending on temperature, in severe light water reactor accidents according to thermodynamic analysis, which treats chemical reactions between boron and cesium. But quality of the thermodynamic data of CsBO (g) used in this thermodynamic analysis is considered to be poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO
(g) used in this thermodynamic analysis is considered to be poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO was performed to obtain reliable thermodynamic quantities of CsBO
was performed to obtain reliable thermodynamic quantities of CsBO (g). The standard enthalpy values of formation of CsBO
(g). The standard enthalpy values of formation of CsBO (g),
(g), 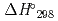 (CsBO
(CsBO ,g), were obtained by the 2nd law and the 3rd law treatments from measured vapor pressures and found to be -700.6
,g), were obtained by the 2nd law and the 3rd law treatments from measured vapor pressures and found to be -700.6  9.8kJ/mol and -698.0
9.8kJ/mol and -698.0  10.6kJ/mol, respectively. These two values agree within the error limits and the evaluated thermodynamic data of CsBO
10.6kJ/mol, respectively. These two values agree within the error limits and the evaluated thermodynamic data of CsBO (g) are considered to be reliable.
(g) are considered to be reliable.